Nombre químico: Cloruro de metoxipolietilenglicol
Nombre en inglés: mPEG-Cloruro o mPEG-Cl
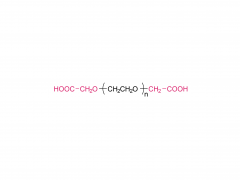
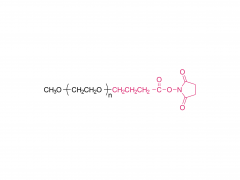
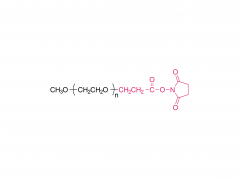
Fórmula molecular: CH₃O-(CH₂CH₂O)ₙ-CH₂CH₂-Cl
Rango de peso molecular: 350 Da a 40.000 Da (comúnmente 1K, 2K, 5K, 10K, 20K)
Propiedades: Sólido blanco o blanquecino (los de bajo peso molecular pueden ser líquidos)
Solubilidad: Fácilmente soluble en agua, DMSO, DMF, THF y otros disolventes polares.
La estructura del mPEG-Cl consiste en una cadena de PEG terminada en grupos metoxi y un grupo clorometilo (-CH₂Cl) en el extremo. Sus características de reacción incluyen:
Reacción de sustitución nucleófila (reacción SN₂):
Puede reaccionar con aminas primarias (-NH₂) para formar enlaces de aminas secundarias estables (-NH-CH₂-PEG).
También puede reaccionar con grupos tiol (-SH) o grupos hidroxilo (-OH) en condiciones alcalinas, pero la eficiencia es relativamente baja.
Condiciones de reacción:
Generalmente se lleva a cabo en una solución tampón con un pH de 8 a 10, como por ejemplo una solución tampón de carbonato.
Se pueden agregar bases orgánicas como trietilamina (TEA) o DIEA para promover la reacción.
Selectividad: En comparación con NHS-PEG o MAL-PEG, mPEG-Cl tiene menor reactividad, pero se puede utilizar para un acoplamiento estable en condiciones específicas.
3. Aplicaciones principales
(1) Bioacoplamiento y modificación de proteínas
Modificación de aminoácidos: Reacciona con el grupo ε-amino de la lisina (Lys) de proteínas, anticuerpos o péptidos para lograr PEG.
Reducir la inmunogenicidad: Prolongar la vida media de los medicamentos y mejorar la estabilidad (como el interferón PEG, los medicamentos de anticuerpos).
(2) Sistema de administración de fármacos
Modificación de la superficie de las nanopartículas: como el PEG de liposomas y micelas poliméricas, mejorando el tiempo de circulación sanguínea.
Acoplamiento de fármacos de moléculas pequeñas: Al unir grupos cloruro a fármacos que contienen aminoácidos, se mejoran la solubilidad y la farmacocinética.
(3) Ciencia de los materiales
Funcionalización de polímeros: Se utiliza en hidrogeles modificados con PEG, materiales de recubrimiento, etc., para mejorar la biocompatibilidad.
Química de superficies: Nanopartículas de oro modificadas, gel de sílice, etc., para reducir la adsorción no específica.